First law of Thermodynamics is based on Energy conservation.
According to this law , work and heat both are the forms of energy and for a cyclic process , net heat transfer is equal to net work transfer.
i.e.,
Qnet = Wnet
The result of first law is based on experiments and following points were also proposed :
(a) Energy can neither be created nor be destroyed.
(b) Internal Energy is energy in storage.
(c)When one form of energy exhaust , it mean it gets transformed to another form of energy.
on adding the above observation following result comes –
∆Q = dE + ∆W
Here ,
∆Q = Heat transfer
∆W = Work transfer
E = internal energy + kinetic energy+ potential energy
If , kinetic energy and potential energy are negligible
Then ,
E = Internal Energy ( U )
i.e., dE = dU
Therefore ,
∆Q = dU + ∆W
This is First law of Thermodynamics.
Internal Energy :
It is the energy in storage of system at molecular level and includes molecular collisions, molecular kinetic energy, chemical energy, nuclear energy and many more microscopic energies.
Internal energy is :
- Property of system
- Point function
- Exact differential
- Cyclic integral is zero
For ideal gas , Internal Energy is the function of temperature only.
Consequences of first law of Thermodynamics:
(a) Heat Transfer is a path function
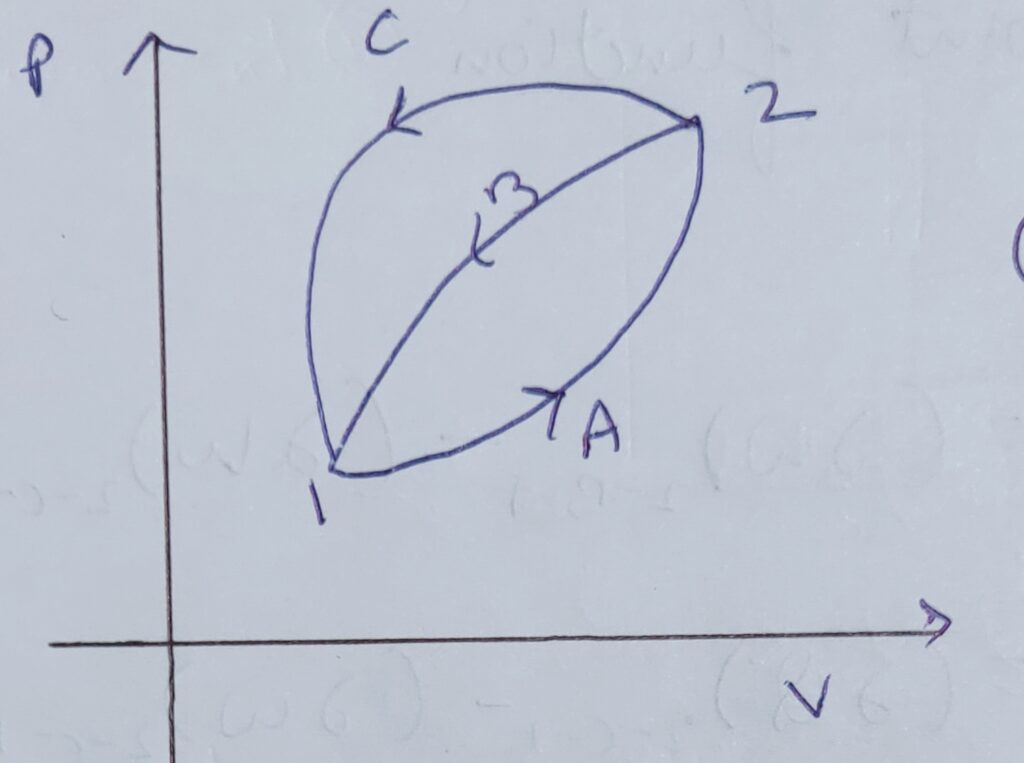
To prove this , consider a P-V diagram as shown above .
For cycle , 1-A-2-B-1
∆Q1-A-2 + ∆Q2-B-1 = ∆ W1-A-2 + ∆W2-B-1
As ,∆Q1-A-2 = ∆ W1-A-2
Therefore , ∆Q2-B-1 = ∆W2-B-1___________eq(1)
For cycle 1- A-2-C-1
∆Q1-A-2 + ∆ Q2-C-1 = ∆W 1-A-2 + ∆W2-C-1
∆Q1-A-2 = ∆W 1-A-2
Therefore , ∆ Q2-C-1 = ∆W2-C-1 _________eq(2)
eq(1) – eq (2)
∆Q2-B-1 – ∆ Q2-C-1= ∆W2-B-1 – ∆W2-C-1 ____eq(3)
Here RHS is not equal to zero
Therefore , LHS will also not be equal to zero.
Conclusion ,
Here the final and initial point of both 2-B-1 and 2-C-1 are same but the heat transfer is unequal .Hence , it is not a point function as it doesn’t depend on initial and final condition.
Therefore heat transfer is a path function.
(b) Internal Energy is a point function :
From equation (3)
∆Q2-B-1 – ∆ Q2-C-1= ∆W2-B-1 – ∆W2-C-1
∆Q2-B-1 – ∆W2-B-1 = ∆ Q2-C-1 – ∆W2-C-1
From first law of thermodynamics
∆Q= dU + ∆W
OR
∆Q-∆W= dU
Therefore
dU2-B-1 = dU2-C-1
Process 2-B-1 and 2-C-1 are different while change in internal energy is same, it means internal energy is a point function.
(c) Energy of isolated system remains constant
For isolated system ,
∆Q = 0
∆W = 0
Therefore , dU will also be zero
i.e ., U = constant
(d) PMM-1 is not possible
Perpetual motion machine of first type is a hypothetical machine which can produce the work continuously without taking any energy from the outside.
It is impossible because it violates the first law of Thermodynamics.



![[Free] Thermodynamics Course With Certification Thermodynamics](https://mechomotive.com/wp-content/uploads/2021/01/SAVE_20210121_225241-218x150.jpg)