Thermodynamics is the branch of physics. It deals with the relationships between heat and forms of energy. Thermodynamics describes how thermal energy is converted to and other forms of energy and how it affects matter. It measures energy, which can be exceedingly complicated. It consists of very large numbers of atoms or molecules interacting in complicated ways.
Heat
Thermodynamics is concerned with several properties of matter such as heat. Heat is a form of energy. It is conserved, but it cannot be created or destroyed. Heat can be transferred from one place to another and can be converted to other forms of energy.
Temperature
The amount of heat transferred depends on the speed and number of atoms. When the atoms move faster, the temperature becomes higher. Temperature is the average kinetic energy expressed in terms of units or degrees. The most commonly used temperature scale is Celsius, Fahrenheit.
Specific heat
The amount of heat required to increase the temperature of a substance by a certain amount is called specific heat. The conventional unit for specific heat is calories per gram per kelvin. The calorie is the amount of heat required to raise the temperature. The specific heat depends on the number of atoms.
Thermal conductivity
Thermal conductivity is the rate at which heat passes through a specified material, expressed as the amount of heat that flows per unit time through a unit area with a temperature gradient of one degree per unit distance. It is denoted as k. The unit for k is watts (W) per meter (m) per kelvin (K).
Heat transfer
Heat can be transferred from one body to another or between a body and the environment by three different means: conduction, convection, and radiation. Conduction is the transfer of energy through a solid material. Conduction between bodies occurs when they are in direct contact, and molecules transfer their energy across the interface.
Convection is the transfer of heat from a fluid medium. Molecules in a gas or liquid in contact with a solid body transmit or absorb heat from that body and then move away, allowing other molecules to move into place and repeat the process. Efficiency can be improved by increasing the surface area with a radiator and forcing the fluid to move over the surface with a fan.
Radiation is the emission of electromagnetic (EM) energy, particularly infrared photons that carry heat energy. All matter emits and absorbs some EM radiation, the net amount of which determines whether this causes a loss or gain in heat.
Entropy
All thermodynamic systems generate waste heat. This waste results in entropy for a closed system. It is a quantitative measure of the amount of thermal energy not available to do work. Entropy in any closed system increases but never decreases. Moving parts produce waste heat due to friction, and radiative heat inevitably leaks from the system. We cannot build an engine that is 100 percent efficient that means we cannot build a perpetual motion machine. But still many people trying to build perpetual motion machines. Entropy is a measure of the disorder or randomness in a closed system, which also inexorably increases.
The four laws of thermodynamics
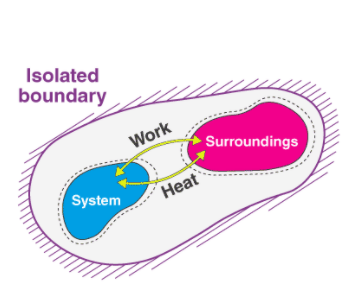
The Zeroth Law states that if two bodies are in thermal equilibrium with some third body, then they are also in equilibrium with each other. This establishes temperature as a fundamental and measurable property of matter.
The First Law states that the total increase in the energy of a system is equal to the increase in thermal energy plus the work done on the system. This states that heat is a form of energy and is therefore subject to the principle of conservation.
The Second Law states that heat energy cannot be transferred from a body at a lower temperature to a body at a higher temperature without the addition of energy. This is why it costs money to run an air conditioner.
The Third Law states that the entropy of a pure crystal at absolute zero is zero.
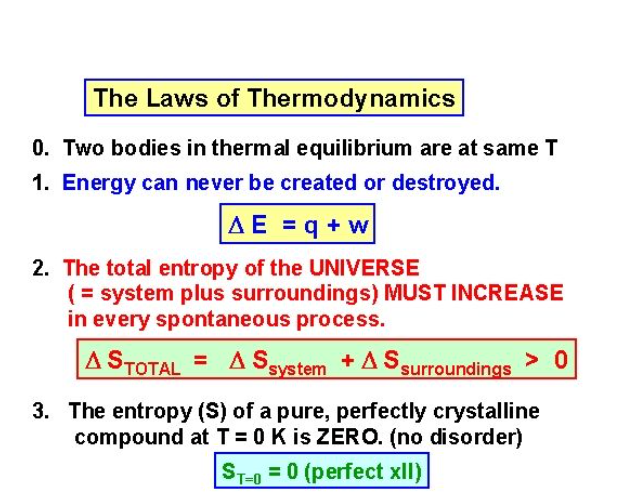
As explained above, entropy is sometimes called waste energy that is unable to do work, and since there is no heat energy at absolute zero, there can be no waste energy. Entropy is also a measure of the disorder in a system, and while a perfect crystal is perfectly ordered, any positive value of temperature means there is motion within the crystal, which causes the disorder. For these reasons, there can be no physical system with lower entropy, so entropy always has a positive value.
The science of thermodynamics has been developed over centuries, and its principles apply to nearly every device ever invented. Its importance in modern technology cannot be overstated.




![[Free] Thermodynamics Course With Certification Thermodynamics](https://mechomotive.com/wp-content/uploads/2021/01/SAVE_20210121_225241-218x150.jpg)