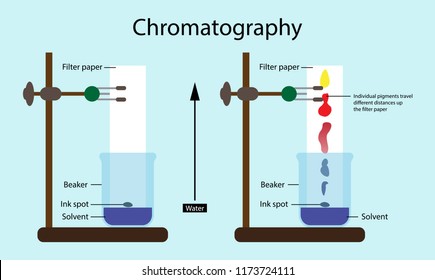
Chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed.
Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound’s partition coefficient result in differential retention on the stationary phase and thus affect the separation.
Chromatography may be preparative or analytical. The purpose of preparative chromatography is to separate the components of a mixture for later use, and is thus a form of purification. Analytical chromatography is done normally with smaller amounts of material and is for establishing the presence or measuring the relative proportions of analytes in a mixture. The two types are not mutually exclusive.
It was first devised in Russia by the Italian-born scientist Mikhail Tsvet in 1900. He developed the technique and coined the term chromatography in the first decade of the 20th century, primarily for the separation of plant pigments such as chlorophyll, carotenes, and xanthophylls. Since these components separate in bands of different colors (green, orange, and yellow, respectively) they directly inspired the name of the technique. New types developed during the 1930s and 1940s made the technique useful for many separation processes.
How does chromatography work step by step?
- A pencil line is draw, and spots of ink or plant dye are place on it. There is a container of solvent, such as water or ethanol.
- The paper is lowered into the solvent.
- As the solvent continues to travel up the paper, the different coloured substances spread apart.
What is the use of chromatography ?
This can used as an analytical tool, feeding its output into a detector that reads the contents of the mixture. It can also used as a purification tool, separating the components of a mixture for use in other experiments or procedures.
What can separated by chromatography?
Paper chromatography has become standard practice for the separation of complex mixtures of amino acids, peptides, carbohydrates, steroids, purines, and a long list of simple organic compounds. Inorganic ions can also readily separated on paper.
What is to become separated from a liquid?
Distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation is a purification process where the components of a liquid mixture are vaporizes and then condensed and isolated. The condensate that is collect is call as distillate.
Can chromatography separate water?
For example, column chromatography can used to separate different water soluble proteins. Here is a video of a lab my students conduct to separate the different pigments present in the ink of a water soluble marker.
What are the two phases of chromatography?
It is a physico-chemical method for separation of compound mixtures, based on the distribution of components between two phases. One of which is stationary (sorbent), and the other, mobile, flowing through a layer of the stationary phase.
What are the 4 types of chromatography?
There are four main types. These are Liquid Chromatography, Gas Chromatography, Thin-Layer Chromatography and Paper Chromatography. Liquid Chromatography is use in the world to test water samples to look for pollution in lakes and rivers.
What factors affect chromatography?
Rf values and reproducibility can affected by a number of different factors such as layer thickness, moisture on the TLC plate, vessel saturation, temperature, depth of mobile phase. Nature of the TLC plate, sample size, and solvent parameters. These effects normally cause an increase in Rf values.
How can we separate cream from milk?
The process of centrifugation can easily used to separate cream from milk. To carry out this the milk is rotate at a very high speed in a centrifuge. The cream being lighter floats over the heavier and skimmed milk.