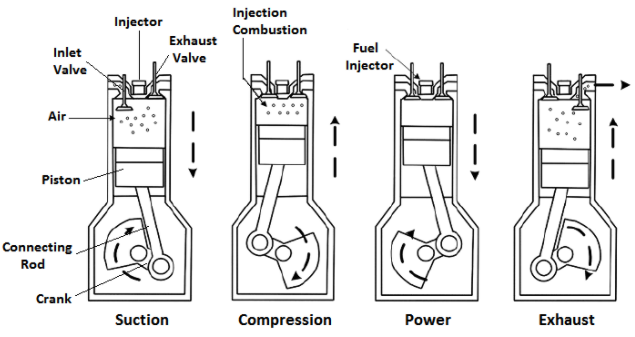
Heat engine is a device which can convert heat energy into mechanical work. In an internal combustion engine the heat energy is released by the process involving burning of fuel in the engine cylinder. The chemical reactions which allows the release of heat energy are quite fast but the time taken in preparing a proper mixture of fuel and air depends mainly upon the nature of the fuel and the method of introducing it into the combustion chamber.
Fuels used in the internal combustion engines are designed in order to satisfy the performance requirement of the engine system in which they are used. Thus, fuel must have certain physical, chemical, and combustion properties, such as:
- High energy density
- Good combustion qualities
- High thermal stability
- Low deposit forming tendencies
- Compatibility with the engine hardware
- Good fire safety
- Low toxicity
- Low pollution
- Easy transferability and onboard vehicle storage
Some of the most basic requirements for an internal combustion engine fuel are:
- The combustion process in the cylinder must take as little time as possible and a maximum amount of heat energy must be released during this period.
- The above requirement must be fulfiled as long as required. Longer operations result in formation of deposits and many side effects such as wear, etc., appear. Deposits combined with other combustion products may cause excessive wear and corrosion of cylinder, piston and piston rings.
In addition to this the products of combustion should not be harmful when they are released to atmosphere. The fuel used should help in reliable and easy starting under ambient conditions. These requirements are met by a number of gaseous and liquid fuels. Solid fuels can be used for internal combustion engines only after gasification.
The natural petroleum oil is the largest single source of internal combustion engine fuels. Other sources of internal combustion engine fuels which are available are hydrocarbon mixtures, coal, oil shales and fermentation products such as ethly alcohol, methanol, etc. If we convert mixture of carbon-monoxide and hydrogen, catalytically then we can form a good fuel for automotive engines.
Coal itself can be converted into hydrocarbon fuel or hydrocarbons form the gases formed in cooking can be separated or coal tar cab be hydrogenated to produce hydrocarbons. But, all these processes are very costly and technical know-how is still not available for competitive production of such fuels.
Oil shales contain a high proportion of waxy and resinous plants which when heated, decompose into oil products very similar to petroleum. Here again, the technology of obtaining fuel at commercial level is not available. The production of alcohol in the present stage is also not competitive and cheaper methods may be available but presently petroleum fuels are the only commercially available fuels.