The P-V diagram or pressure volume diagram or pressure volume loop makes the comparative study of thermodynamic variables very simple.
The P-V diagram is very useful to study the changes taking place in one component corresponding to another component.
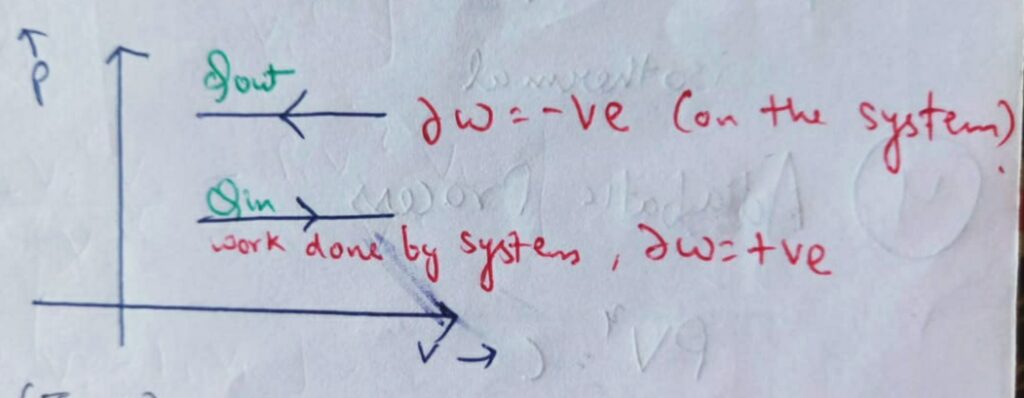
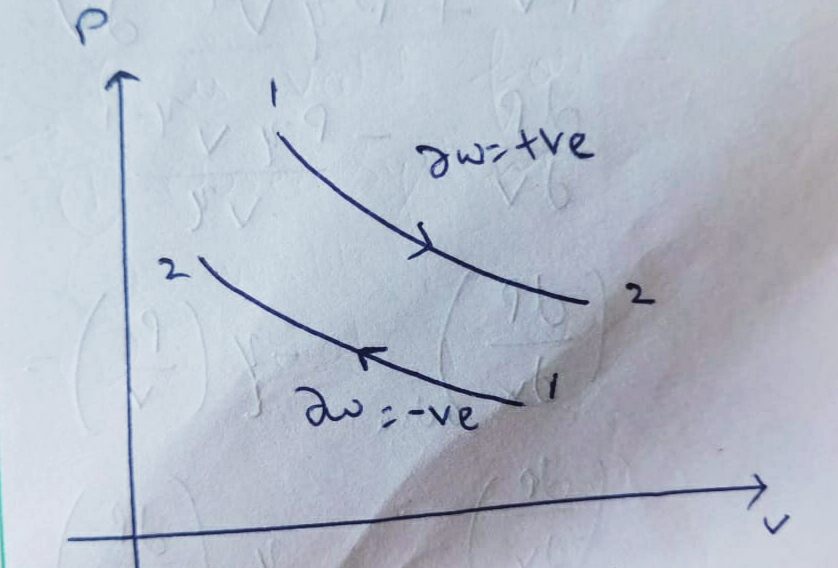
Area under the P-V diagram represents the net work transfer in any thermodynamic process.
P-V diagrams for the different thermodynamic processes are given below :
We have discussed about various thermodynamic processes and their displacement work in the previous post
(a) Isochoric Process :
As we know that in an Isochoric process , volume remains constant ;
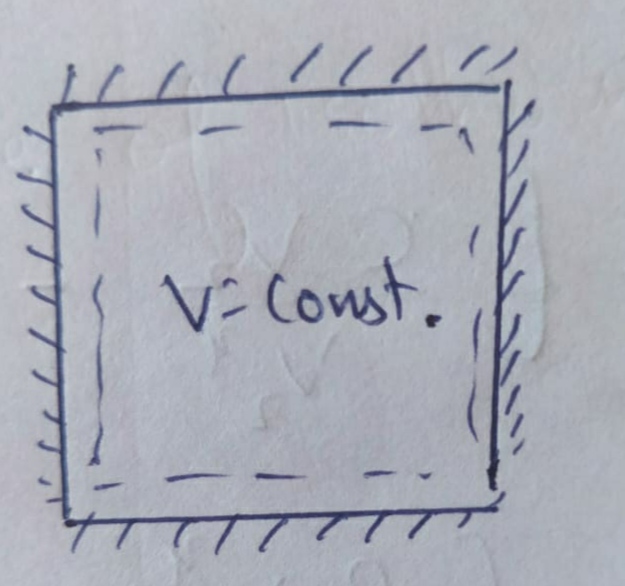
Therefore , PV diagram for Isochoric Process will be straight line.
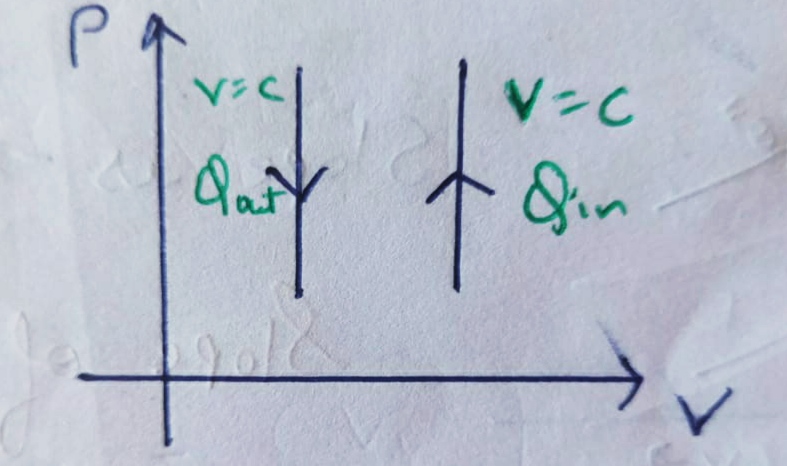
and from it we can conclude that the change in Volume is zero , therefore it’s work done will also be zero.
(b) Isobaric Process :
In the Isobaric Process , presure remains constant ;
And therefore the P-V diagram of Isobaric Process will be straight line with slope zero .
(c) Isothermal process:
In the Isothermal process, the temperatute remains constant ;
i.e., T = constant
Therefore ,
PV = MRT
PV = c ___________eq (1)
Differentiating the above equation , we get
PdV + VdP = 0
OR , PdV = -VdP
(dP/dV ) = -(P/V )
Here we can see that the slope of the PV diagram of Isothermal process is negative.
Therefore it’s PV diagram will be like:
Note : Slope of isothermal process is always negative .
(d) Adiabatic process :
In the Adiabatic process, the net heat transfer is zero ;
and PV¥ = constant ( here ¥= gamma = adiabatic index)
On differentiating the above equation
We get ,
(dP/dV ) adiabatic = ¥ ( – P/V )
Here , we can see that the slope of Adiabatic process is also negative but greater in magnitude than that of isothermal process .
Because ¥ >1
Therefore it’s P-V diagram will be like :
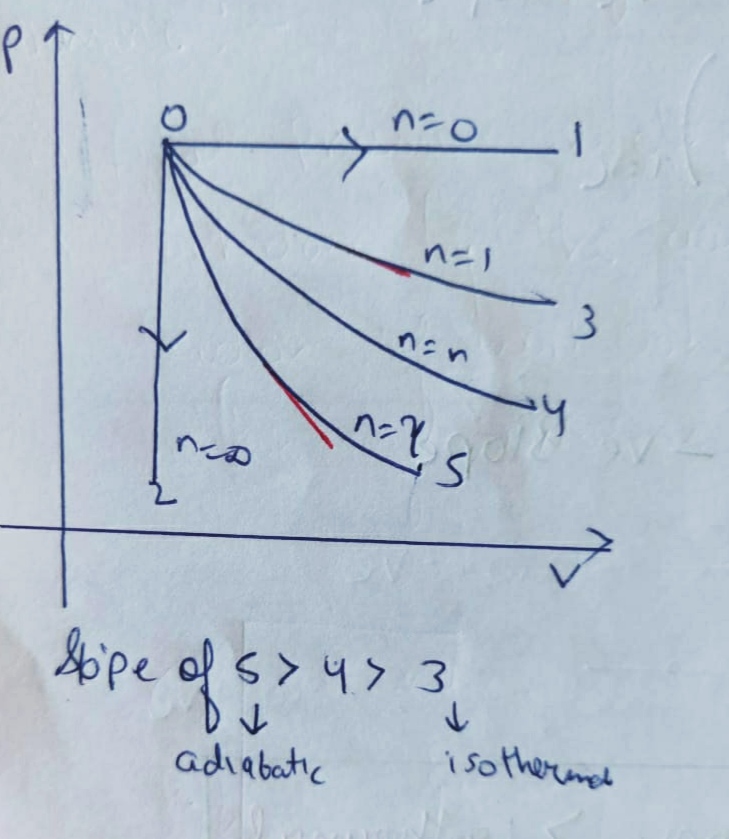
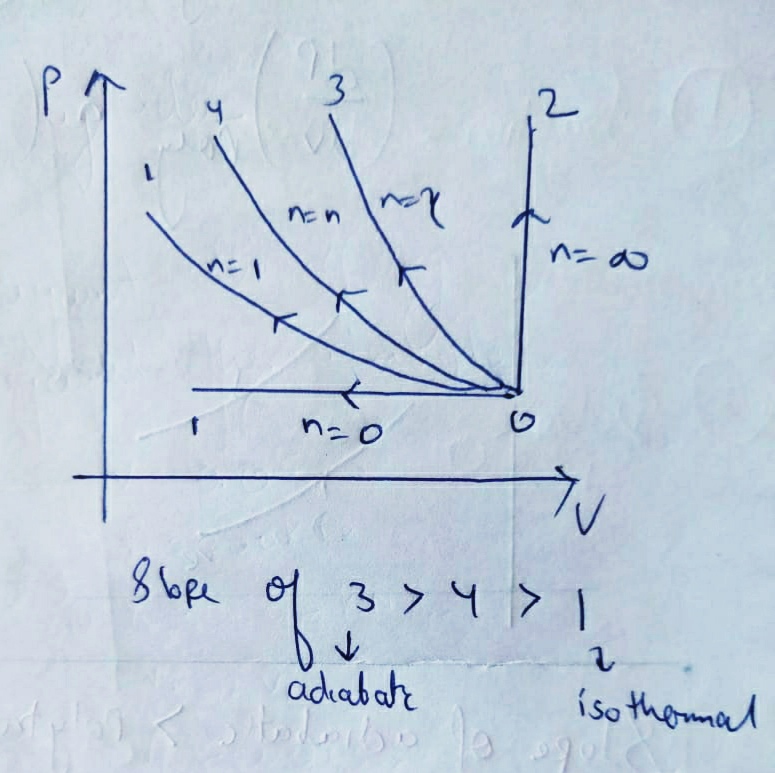
(e) Polytropic process :
The Polytropic process obeys the following relation :
PVn = C
Where ,
P = pressure
V = Volume
N = polytropic index
C = constant
On differentiating the above equation , we get
(dP/dV) Polytropic = N ( -P/ V )
here , we can conclude that the slope of PV diagram of Polytropic process is negative and greater than isothermal process but less than Adiabatic process.
Because , 1 < N < ¥
Therefore the P-V diagram of Polytropic process will be like :
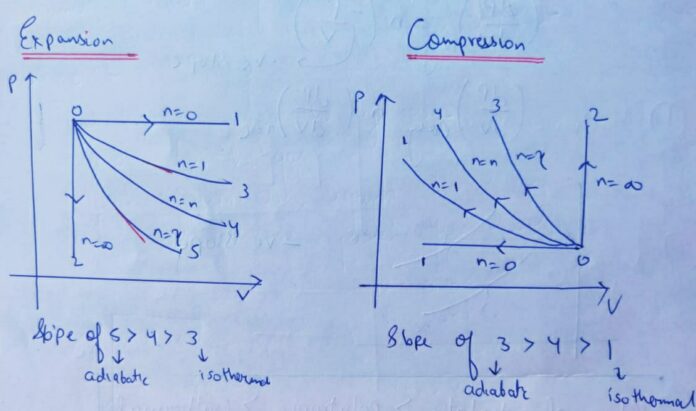
Relationship between Slope of P-V diagram of different thermodynamic processes :
Slope of Adiabatic
>
Polytropic
>
ISOTHERMAL
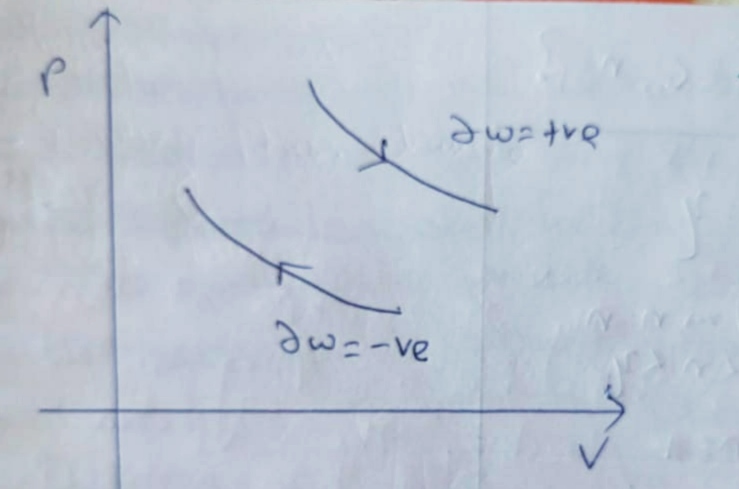
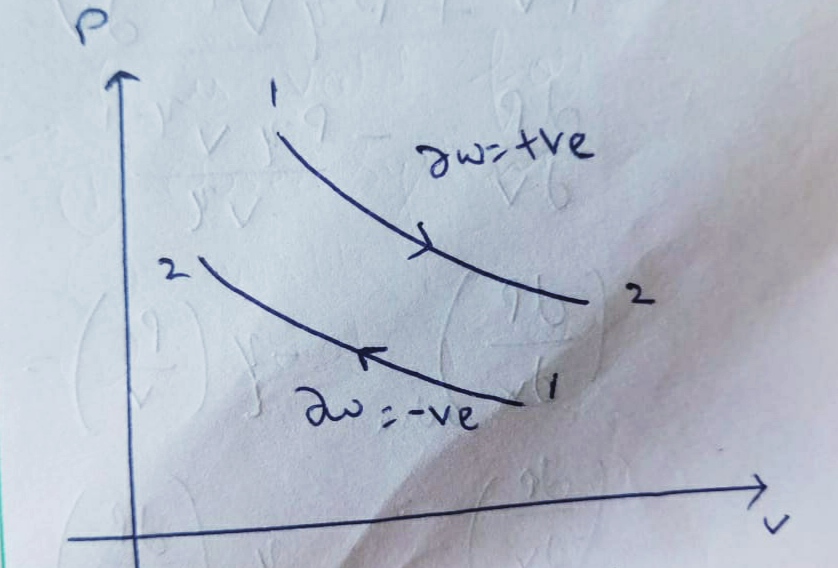
Expansion :
Compression :



![[Free] Thermodynamics Course With Certification Thermodynamics](https://mechomotive.com/wp-content/uploads/2021/01/SAVE_20210121_225241-218x150.jpg)