Thermodynamics is a branch of physics that deals with heat, work, and temperature and their relation to energy, radiation, and physical properties of matter. The behavior of these quantities is governed by the four law of thermodynamics which convey a quantitative description using measurable macroscopic physical quantity and but may be explained in terms of microscopic constituents by statistical mechanics.
Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.
Laws of Thermodynamics:
- 0th Law of Thermodynamics
- 1st Law of Thermodynamics.
- 2nd Law of Thermodynamics.
- 3rd Law of Thermodynamics.
Description of laws:
0th Law:
The Zeroth Law states that if two systems are in equilibrium with a third system, the two original systems are in thermal equilibrium with each other.
1st Law:
The First Law states that energy can be converted from one form to another with the interaction of heat, work and internal energy, but it cannot be created nor destroyed, under any circumstances.
2nd Law:
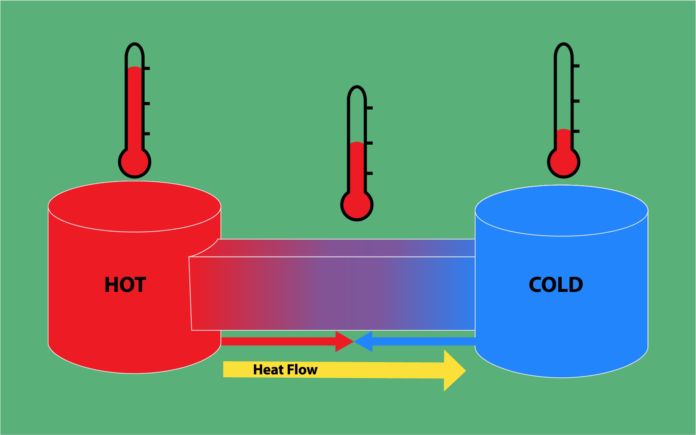
The Second Law states that the state of entropy of the entire universe, as an isolated system, will always increase over time. The second law also states that the changes in the entropy in the universe can never be negative.
3rd Law:
The 3rd law will essentially allow us to quantify the absolute amplitude of entropies. It says that when we are considering a totally perfect (100% pure) crystalline structure, at absolute zero (0 Kelvin), it will have no entropy (S). Note that if the structure in question were not totally crystalline, then although it would only have an extremely small disorder (entropy) in space, we could not precisely say it had no entropy.



![[Free] Thermodynamics Course With Certification Thermodynamics](https://mechomotive.com/wp-content/uploads/2021/01/SAVE_20210121_225241-218x150.jpg)
