Most of the thermodynamic relations are valid under certain conditions , like
PV¥ = constant , valid only for reversible adiabatic process
Similarly ,
∆W = mRT1 ln ( V2 / V1 ) , valid only for
- closed system
- Reversible process
- Ideal gas
Therefore it becomes important to know about these parameters in Thermodynamics .
What is ideal gas?
Ideal gas is the gas for which –
(a) The intermolecular forces i.e., molecular attraction and molecular repulsion forces are negligible.
(b) Volume occupied by the molecule becomes negligible compared to the container volume.
(c) all gas laws are followed in all conditions
(d) temperature remains high and pressure low.
At low pressure and high temperature all the gas start to behave as ideal gas.
Example of ideal gas :
In reality no gas is ideal gas , but under certain conditions ( high temperature and low pressure ) real gases like nitrogen , oxygen and some other gases behaves like ideal gas.
Note : water vapour is considered as ideal gas while steam is not considered as ideal gas
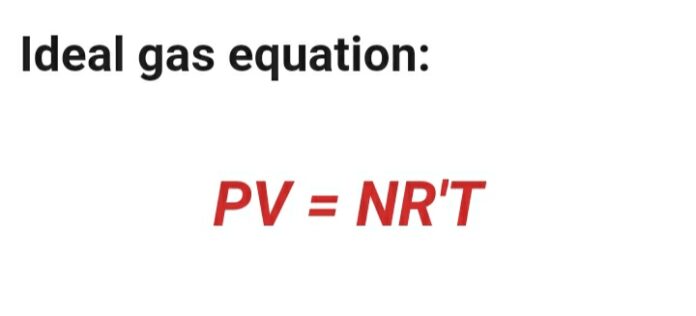
Ideal gas equation:
Pv = nR’T
Here ,
P = Presure
V= Volume
N = Number of moles
R’ =Universal gas constant
T = Temperature
It can also write like :
Pv=mrt
Where ,
M = mass ( in Kg)
R = Characteristic Gas constant (KJ/Kg -Kelvin )
Relationship between R’ and R :
R = R’/ (MOLECULAR weight)
R’ stands for Universal gas constant whose value remains fixed always and it is 8.314 KJ/ K-mol.Kelvin
Whereas,
R stands for characteristic gas constant and it’s value is different for different gas.
For example :
For air –
Molecular weight = 29 g/mol = 29 × 10-3 Kg/mol
We know that , R = R’/Molecular weight
Rair = 8.314 / 29 = 0.287
Rair = 0.287 KJ/ Kg – Kelvin
Similarly ,
Rwater vapour = 0.416 KJ/ Kg -Kelvin



![[Free] Thermodynamics Course With Certification Thermodynamics](https://mechomotive.com/wp-content/uploads/2021/01/SAVE_20210121_225241-218x150.jpg)