Thermodynamics is basically the science of energy transfer and its effect on the physical properties of a substance. We can broadly classify thermodynamics into Classical thermodynamics and Statistical Thermodynamics. In this blog, we will deal with the concepts of classical thermodynamics.
Thermodynamic System and Surrounding
A Thermodynamics system is a region in space under which the attention is focused for the study and other than a system whatever is in the universe is called surrounding. The thermodynamic system and surrounding are separated by Boundary. The boundary can be fixed, movable, or imaginary.
System + Surrounding = Universe
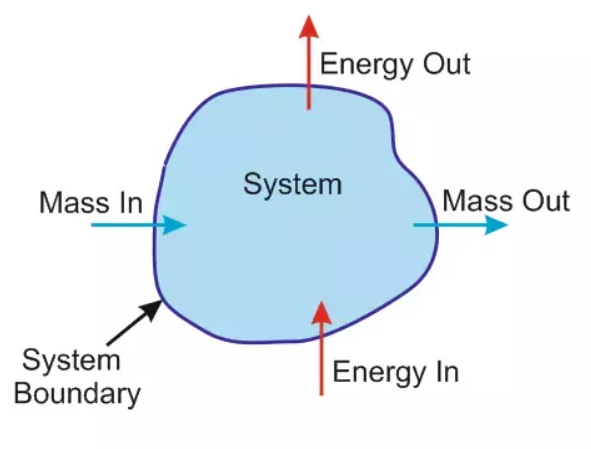
There are three types of systems:
- Open System
- Closed System
- Isolated System
| System | Mass Interaction | Energy Interaction |
| 1. Open System | yes | yes |
| 2. Closed System | no | yes |
| 3. Isolated System | no | no |
| System | Examples |
| Open System | Nozzle, diffuser, turbine, compressor, IC Engine |
| Closed System | Closed Container |
| Isolated System | Thermus Flask, Universe |
Thermodynamic Properties, Processes and Cycles
Thermodynamics Properties:
Properties are the coordinates to describe the state of a system. In simple language Properties are the characteristics of the state of a system. Every system has some characteristics by which their physical condition may be described like volume, temperature, pressure, etc.
There are two types of thermodynamic properties:
- Intensive Properties
- Extensive Propeties
Intensive Properties:
Intensive properties are independent of amount of substance. Examples of Invensive properties: Pressure, temperature, specific volume etc.
Extensive Properties:
Extensive Properties depends on amount of substance. Examples of Extensive properties: mass, volume, energy etc. The Ration of two extensive properties is always intensive. All the specific extensive properties are Intensive.
Thermodynamic Processes:
Any operation in which one or more of the properties of a system changes is called a change of state. The change in state when the path is completely specified is called the Process. For example constant volume process (Isochoric Process), constant pressure process (Isobaric Process), constant temperature process (Isothermal Process) etc.
Thermodynamic Cycle:
Thermodynamic cycle can be defined as series of state changes such that the final state is identical with the initial state.
Thermodynamic Equilibrium
A system will be in a state of thermodynamic equilibrium only if mechanical, chemical, and thermal equilibrium exists. In Mechanical equilibrium the net force should be zero, In chemical equilibrium, no reaction should occur and in thermal equilibrium, there is no transfer of heat from one body to another (Temperature=constant).
Reversible and Irreversible Process
Reversible Process can be reversed without any effect on the system and surrounding. If the process is against conservative force then, it is reversible and if it is against non-conservative force then it is irreversible.
Quasistatic Process
It is a imaginary process that takes infinite time to complete such that system remains in equilibrium throughout the process and process becomes reversible. All reversible processes are quasistatic but all quasistatic processes might not be reversible. Frictionless quasistatic process is reversible.



![[Free] Thermodynamics Course With Certification Thermodynamics](https://mechomotive.com/wp-content/uploads/2021/01/SAVE_20210121_225241-218x150.jpg)