First law of Thermodynamics is based on Energy conservation . In the previous post we studied about two forms of thermodynamics .
In first law of Thermodynamics for open system we described the relation,
m{ h1 + v1 2 /2 + gZ1 } + ∆Q/∆t = m{ h2 + v2 2 /2 + gZ2} + ∆W/∆t
This relation is Steady Flow Energy Equation or SFEE.
With the help of this equation , we can find out the value of work transfer for open system.
Let’s assume the kinetic energy and potential energy negligible
Now from SFEE ,
m{ h1 + v1 2 /2 + gZ1 } + ∆Q/∆t = m{ h2 + v2 2 /2 + gZ2} + ∆W/∆t
then ,
mh1 + ∆Q/∆t = mh2 + ∆W/∆t
∆Q/∆t = m (h2 – h1) + ∆W/∆t
∆Q/∆t = m dh + ∆W/∆t
Now , considering it for per unit mass
Then ,
∆Q/m = dh + ∆W/∆m______________eq(1)
we know that ,
∆Q = dH – VdP , where V = Total volume
Dividing this relationship by ∆m
∆Q/∆m = dH/∆m – VdP/∆m
Here , dH/∆m = dh = specific enthalpy
& , V/∆m = v = specific volume
So ,
∆Q = dh – vdP__________eq(2)
Comparing eqn (1) and(2)
We get ,
∆W/∆m = – int(vdP)
This is the work transfer for open system .
Graphical representation of work transfer for open system :
For open system :
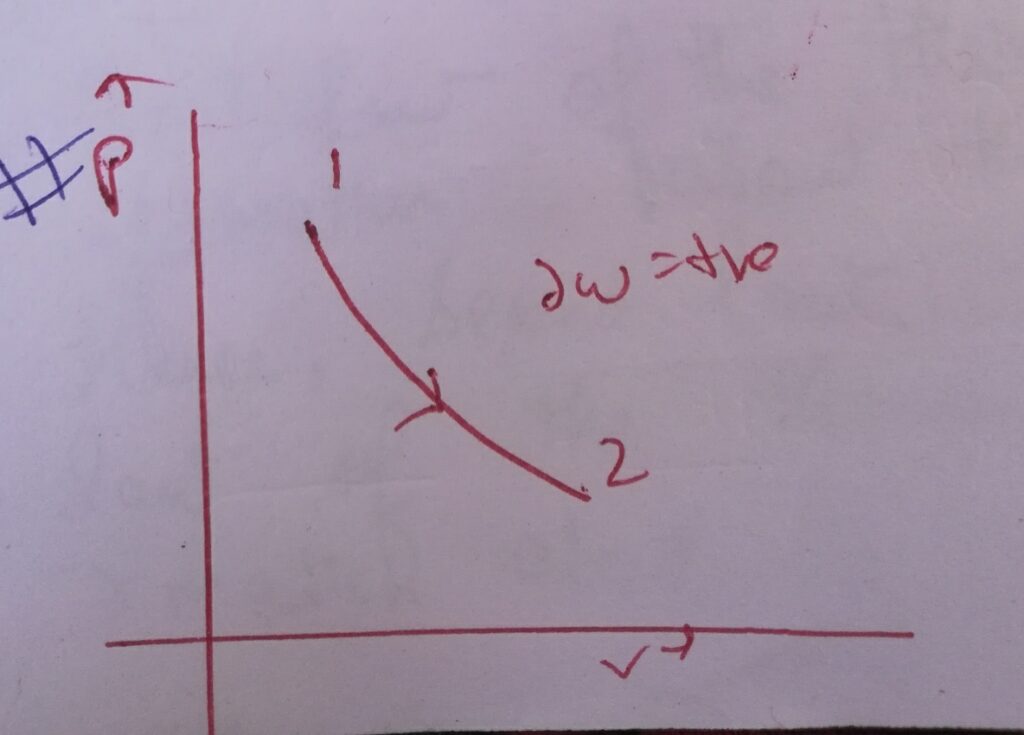
Case (I) :
∆W= – int( vdP) , where dP = +ve
∆W=-ve ,
Then graphically it is represented as –
Here the slope of the PV curve is increasing .
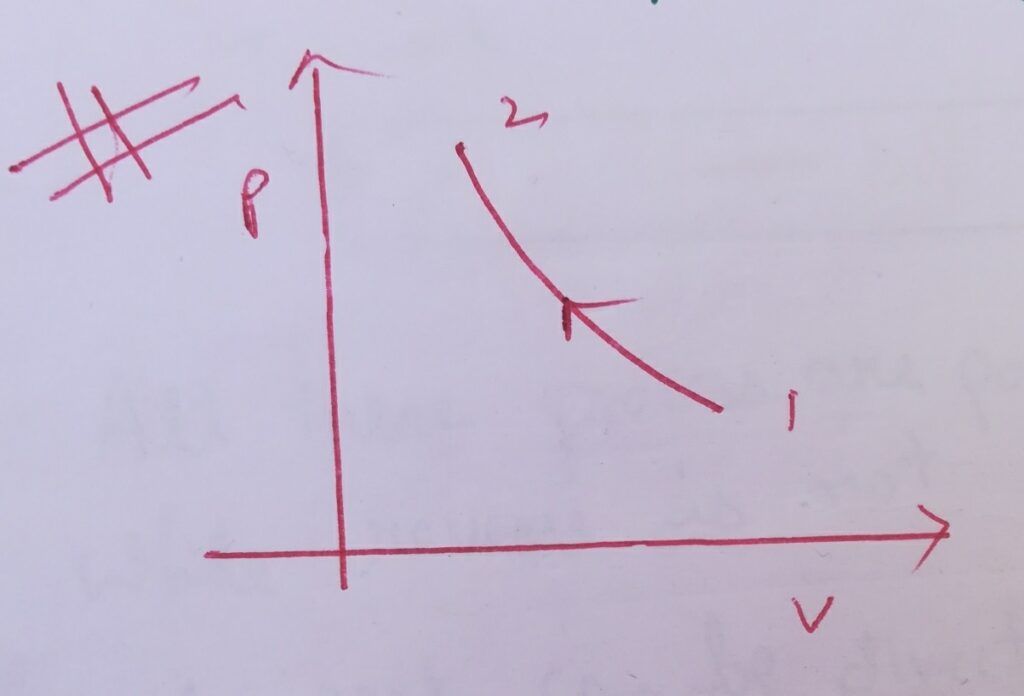
Case (II) :
∆W= – int( vdP) , where dP = -ve
∆W = +ve
Then graphically it is represented as –
Here the slope of the PV curve is decreasing .
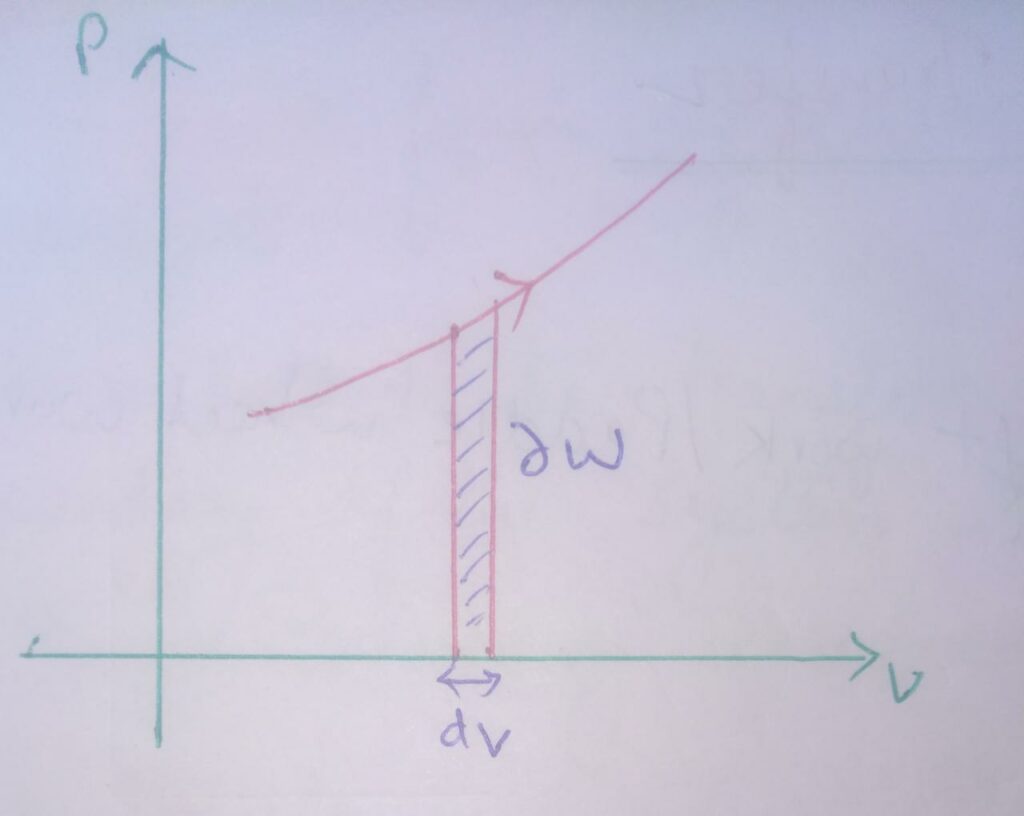
Graphical representation of work transfer for closed system :
∆W = PdV , here dV = -ve
Therefore , ∆W = -ve




![[Free] Thermodynamics Course With Certification Thermodynamics](https://mechomotive.com/wp-content/uploads/2021/01/SAVE_20210121_225241-218x150.jpg)