In the thermodynamics , the phenomenon of expansion and compression plays an important role .
Because thermodynamic expansion or compression decides the sign of work i.e.,
•If the expansion takes place then work is done by the system and the work done will be positive
• If the compression takes place then work is done on the system and the work done will be negative
But what if both expansion and compression occurs simultaneously in equal magnitude ?
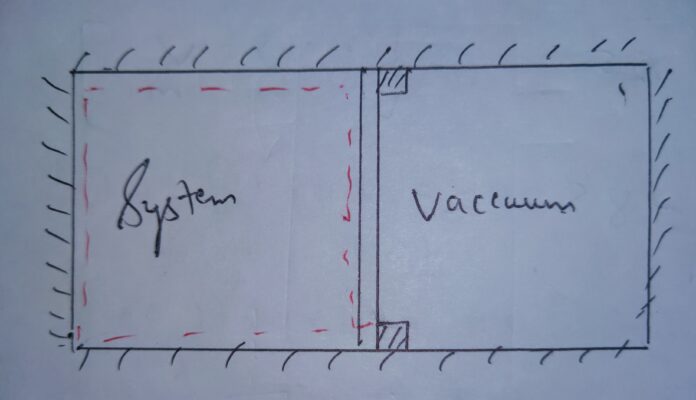
This possibility happens in case of Free Expansion , where the expansion takes place against the vacuum .
What is Free Expansion ?
Free Expansion is the thermodynamic expansion which takes place against the vacuum .
See the following image to understand it in better way .
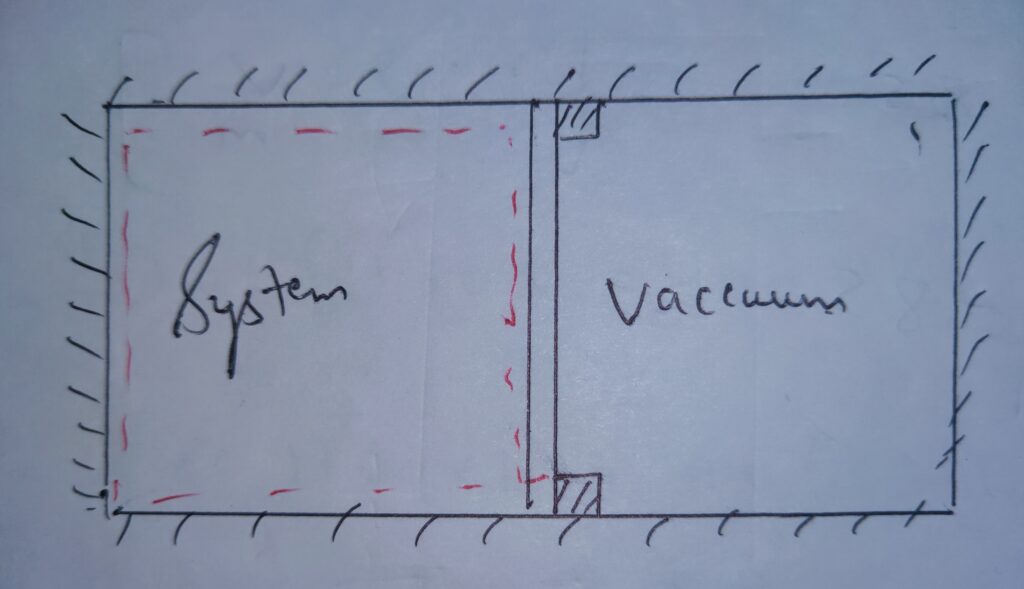
In the image it is clear that the system expands against the vacuum and undergoes the free expansion .
In the free expansion as any external agent didn’t interfere and magnitude of expansion and compression is equal .
Therefore, the work transfer in free expansion is zero.
∆W = 0 ( net work transfer )
Free Expansion is a Fast Process and as we now that all the fast process are irreversible process .
Therefore , Free Expansion is fast and irreversible process.
Impact on Internal energy , Temperature and Enthalpy due to Free Expansion :
(a) Internal energy :
Initially , system is in static position and then it start to move . Hence , part of internal energy of the system changes to the kinetic energy .
In between the process , Internal Energy decreases and at the end of the process, if the losses are negligible then kinetic energy restore to internal energy.
The variation of Internal energy with respect to Process is
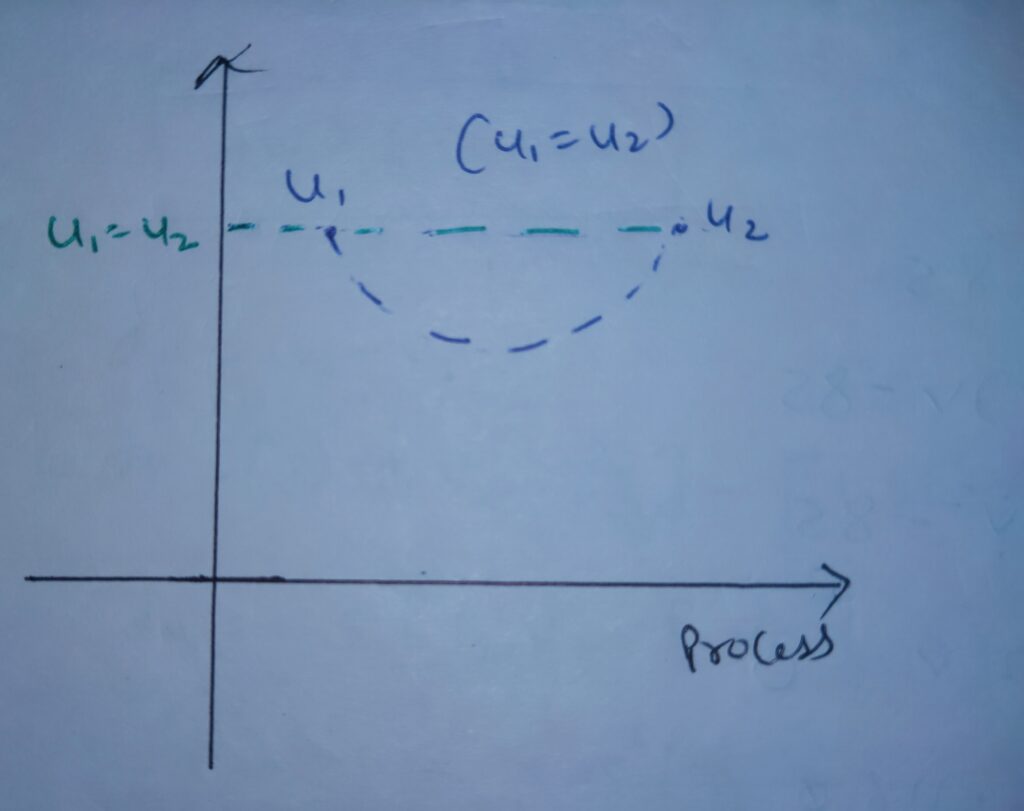
Hence final and initial internal energy of the free expansion remains same .
dU = 0
Or
Ufinal = Uinitial
(b) Temperature :
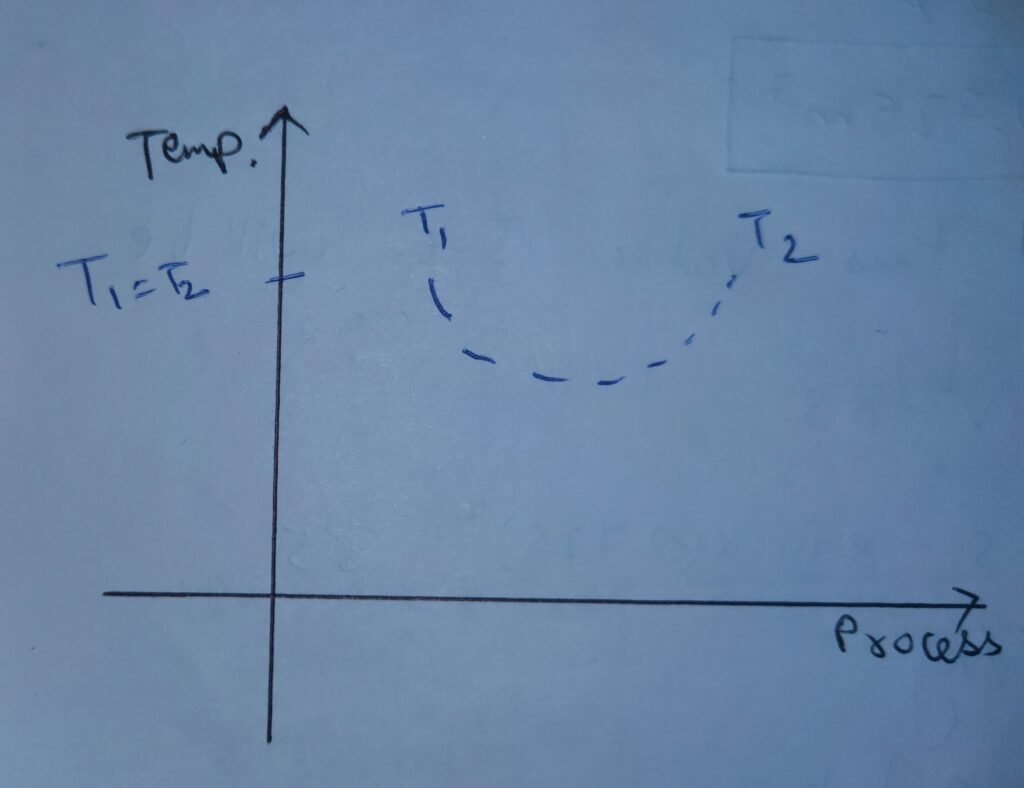
In free expansion internal energy first decreases and then increases and it’s net change remains zero .
Temperature is the measure of energy at microscopic level i.e., internal energy .
And for ideal gas , Internal energy is the function of temperature only .
Therefore , Temperature also shows the similar response like internal energy in case of free expansion.
The variation of temperature with respect to Process is
(c) Enthalpy :
As we know that Enthalpy is denoted by h , and
h = U + PV
In this term
U = function of temperature
PV = mRT = function of temperature
Therefore
Enthalpy is also the function of temperature .
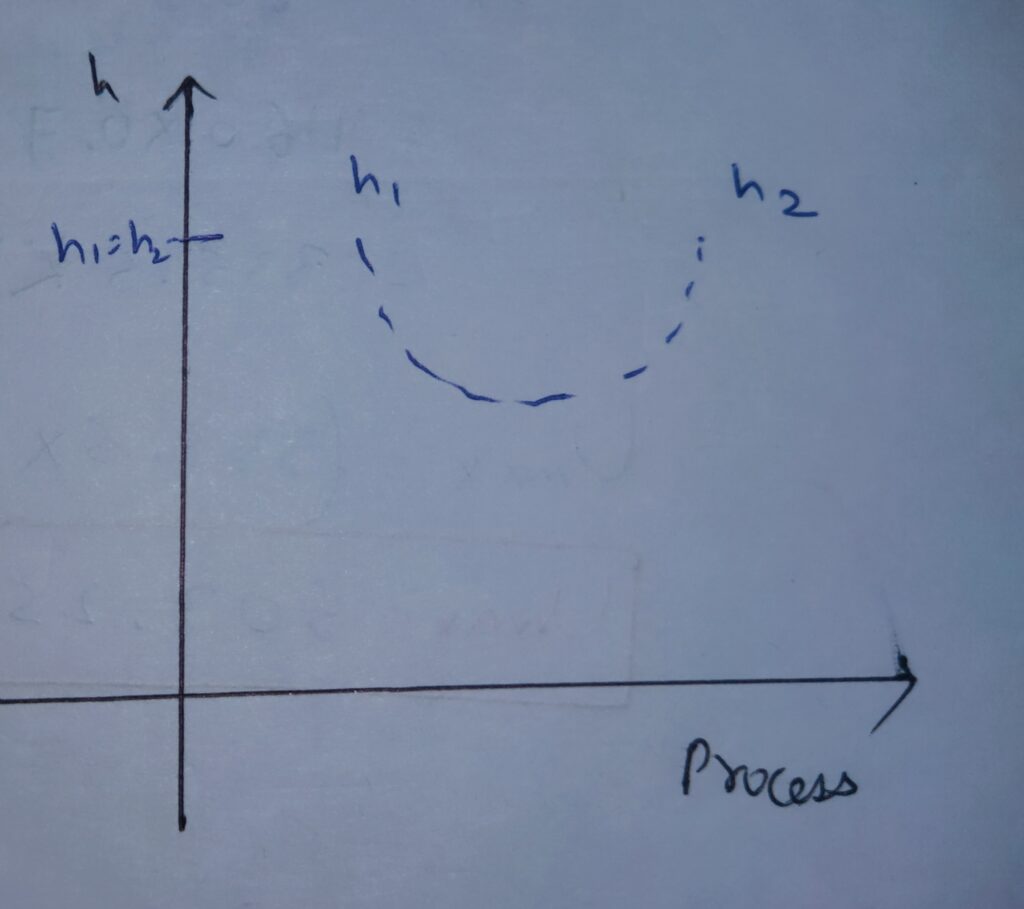
Likewise, Internal energy and temperature , Enthalpy also obeys the same variation with Process.
Enthalpy first decreases and then increases and net change remains zero.



![[Free] Thermodynamics Course With Certification Thermodynamics](https://mechomotive.com/wp-content/uploads/2021/01/SAVE_20210121_225241-218x150.jpg)